Efficacy and safety of abrocitinib for atopic dermatitis: results of a phase III trial
European Academy of Dermatology and Venereology Congress 2019
Following the approval and adoption of dupilumab in the United States (US), Europe and Japan, several agents with novel mechanisms of action are now being reviewed as alternative treatment options for patients with atopic dermatitis (AD).
At the 28th Annual Congress of the European Academy of Dermatology and Venereology (EADV), held 9-13 October 2019 in Madrid, Spain, researchers presented new data on several investigational therapies evaluated in phase II and III randomised controlled trials (RCTs). Among the therapies discussed was abrocitinib, a selective oral Janus kinase (JAK) inhibitor that targets JAK1. At EADV 2019, Professor Eric L Simpson reported on the efficacy and safety of abrocitinib therapy for patients with moderate-to-severe AD enrolled in the phase III JADE MONO-1 study.1 In this week’s commentary, we present their findings.
JADE MONO-1 study
JADE MONO-1 is an international multicentre, double-blind, placebo-controlled RCT (NCT03349060). Eligible patients were adolescents and adults (≥12 years of age) with AD for 1 year or more, inadequately controlled by topical medication or requiring systemic therapy. All patients had moderate-to-severe AD, measured by:
- Investigator’s Global Assessment (IGA) score ≥3
- Eczema Area and Severity Index (EASI) score ≥16
- Body surface area (BSA) coverage ≥10%
- Peak Pruritus numerical rating scale (NRS) score ≥4
The 387 randomised patients underwent 12-week dosing with a 4-week follow-up, and were allocated to either:
- Abrocitinib 200 mg once daily (QD) (n = 154)
- Abrocitinib 100 mg QD (n = 156)
- Placebo QD (n = 77)
The mean age (± standard deviation [SD]) across groups was 32.5 ± 16.0 years and 56.8% of patients were male. Co-primary endpoints included IGA and ≥75% improvement in EASI score from baseline (EASI-75) responses at Week 12.
Efficacy of abrocitinib
In adults and adolescents, abrocitinib 200 mg or 100 mg once daily significantly improved signs and symptoms of moderate-to-severe AD compared with placebo. IGA and EASI-75 responses to the abrocitinib doses were significantly greater relative to placebo as early as Week 2 and continued to improve until Week 12 without plateau (Figure 1).
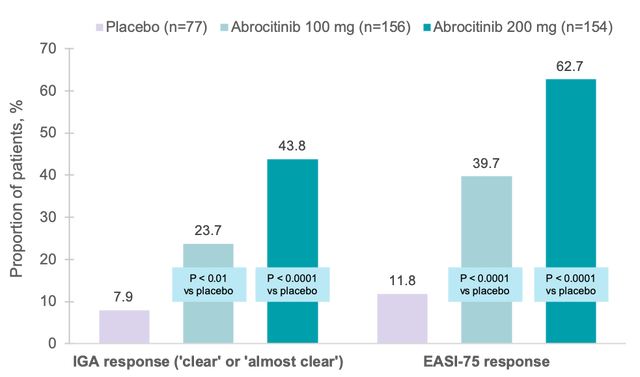
Figure 1. Co-primary endpoint patient responses at Week 12.
EASI-75, ≥75% improvement in EASI score from baseline; IGA, Investigator’s Global Assessment.
Significant early (i.e. 1 day after treatment initiation) reduction in pruritus severity was observed and continued to Week 12. In the abrocitinib groups, the Peak Pruritus NRS response (≥4-point improvement), a key secondary endpoint, was significantly greater at Weeks 2, 4 and 12 when compared to placebo (Table 1). Other secondary endpoint measurements, including the proportion of patients achieving ≥50% (EASI-50) and ≥90% (EASI-90) improvements in EASI score from baseline at Week 12 and the Scoring Atopic Dermatitis (SCORAD) percentage change at all timepoints, were also improved in the abrocitinib groups relative to the placebo group.

Table 1. JADE MONO-1 secondary endpoints.
EASI-50, ≥50% improvement in EASI score from baseline; EASI-75, ≥75% improvement in EASI score from baseline; EASI-90, ≥90% improvement in EASI score from baseline; EASI, Eczema Area and Severity Index; NRS, numerical rating scale; SCORAD, Scoring Atopic Dermatitis.
aNRS ≥4-point improvement.
bP ≤0.001 versus placebo.
cP ≤0.0001 versus placebo.
Safety of abrocitinib
Abrocitinib was well tolerated with an acceptable short-term safety profile (Table 2). The rate of treatment discontinuations because of treatment-emergent adverse events was low in both treatment groups compared to the placebo group. There were no deaths, malignancy or major adverse cardiovascular events. Clinical laboratory evaluations of haemoglobin, neutrophils and lymphocytes showed no clinically significant changes; however, dose dependent changes in lipid levels were observed, with ~10% increase in low-density lipoprotein and ~20% decrease in high-density lipoprotein.

Table 2. JADE MONO-1 summary of adverse events.
AE, adverse event; TEAE, treatment-emergent adverse event.
In summary, encouraging new data from the JADE MONO-1 study has shown that abrocitinib may be a promising novel treatment option for adults and adolescents with moderate-to-severe AD.
Action Eczema
To find out more about the mechanisms of action and use of established and emerging topical and systemic therapies in adult patients with AD, explore our online CME courses:
To test your knowledge of eczema treatment and management as part of patient-centred care, try our ‘Self-assessment in atopic dermatitis’ and ‘Case challenge: 32-year-old woman with worsening skin symptoms’ modules.
Interested in reading more about the AD research presented at EADV 2019? Sign up to Action Eczema and be notified when our EADV 2019 Congress Report is available online for viewing and CME credit.
References
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: results from the phase 3, JADE MONO-1 study. Presented at the European Academy of Dermatology and Venerology (EADV) 2019. 9-13 October 2019; Madrid, Spain. Oral session.