
EADV 2019: Dupilumab plus topical corticosteroids for atopic dermatitis
European Academy of Dermatology and Venereology Congress 2019
Researchers from around the world gathered last month in Madrid, Spain to share their AD research at the 28th Annual Congress of the European Academy of Dermatology and Venereology (EADV), held 9–13 October 2019.
New efficacy and safety data for atopic dermatitis (AD) treatments, including mono- and combined therapies assessed in different patient populations, was presented at the EADV Congress. This included assessments of the biologic dupilumab and JAK inhibitors baricitinib, abrocitinib and upadacitinib, as well as topical tacrolimus and topical corticosteroids (TCS).
At the EADV Congress, Dr Marjolein de Bruin-Weller reported on an investigation into rescue therapy usage in patients with AD being treated with both dupilumab and TCS. Their analysis included patients enrolled in the LIBERTY AD CHRONOS and LIBERTY AD CAFÉ phase 3 trials. In this week’s commentary, we present their findings.
Dupilumab and topical corticosteroids
Topical steroids, the most common treatment for AD flares, are available in different potencies and can reduce redness, inflammation and itching. Dupilumab is a fully monoclonal antibody that blocks the shared receptor component for interleukin (IL)-4 and IL-13, thus inhibiting signalling both IL-4 and IL-13. IL-4 and IL-13 are key type 2 inflammatory cytokines in the pathophysiology of AD.1
Dupilumab is approved for subcutaneous administration every 2 weeks for use in patients aged 12 years and older with moderate-to-severe AD who are candidates for systemic therapy in the EU, for the treatment of patients aged 12 years and older with moderate-to-severe AD inadequately controlled with topical prescription therapies or when those therapies are not advisable in the USA, and for the treatment of adult AD patients not adequately controlled with existing therapies in Japan.
LIBERY AD CHRONOS and LIBERTY AD CAFÉ trial design
Researchers sought to determine the requirement for rescue therapy in patients with AD being treated with dupilumab and TCS in the phase 3 trials LIBERTY AD CHRONOS (NCT02260986) and LIBERTY AD CAFÉ (NCT02755649). The study designs are illustrated in Figure 1. First line rescue treatment was potent and very potent TCS. If patients were unresponsive to TCS after a period of 7 days, rescue was escalated to systemic immunosuppressants.
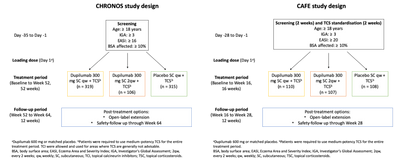
Systemic rescue treatment included any locally approved treatment (e.g. cyclosporine a, azathioprine, methotrexate, mycophenolate mofetil, phototherapy etc). In both trials, patients who received topical rescue treatment were considered treatment failures and were allowed to continue treatment. Patients who received systemic medication were discontinued from study treatment.
Rescue treatment after dupilumab plus topical corticosteroids
Measurements taken at baseline included:
- Total Eczema Area and Severity Index (EASI) score
- Investigator’s Global Assessment (IGA) score
- Body surface area (BSA) affected by AD (%)
- Peak Pruritus Numerical Rating Scale (NRS) score
- Patient-Orientated Eczema Measure (POEM)
- Dermatology Life Quality Index (DLQI) score
- Total SCORing Atopic Dermatitis (SCORAD) score
Baseline disease characteristics were similar across treatment groups between patients requiring and patients not requiring rescue medication in the CHRONOS trial. The efficacy and safety results are presented in Table 1 and Table 2, respectively.
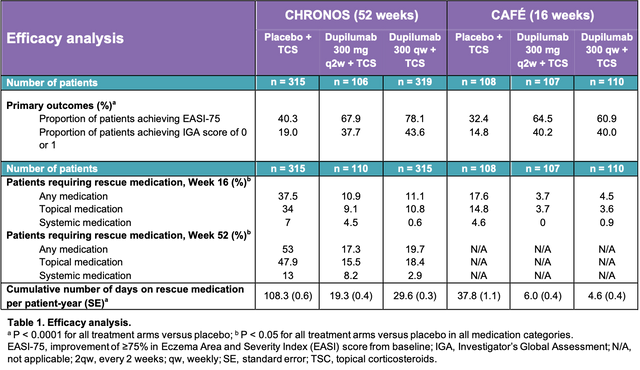

The majority of adult patients with moderate-to-severe AD treated with dupilumab plus TCS did not require additional treatment with either higher potency TCS or systemic rescue medication. Treatment with dupilumab plus TCS significantly reduced the cumulative number of days on rescue medication in patients, compared with placebo. Dupilumab showed improvement in signs and symptoms in AD with an acceptable safety profile. No new safety signals were observed during these trials compared with previous phase 3 findings.
Action Eczema
To find out more about how therapies work for the treatment and management of AD, explore our online CME courses on the mechanisms of action:
To improve your knowledge of eczema treatment as part of patient-centred care, visit our CME course ‘Atopic dermatitis treatment in adults: applying guidelines and new data’, or try our case challenges:
Interested in reading more about the AD research presented at EADV this year? Sign up to Action Eczema and be notified when our EADV 2019 Congress Report is available online for viewing and CME credit.
References
- Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.